Mair Zamir
Professor of Applied mathematics (emeritus)
Professor of Medical Biophysics (emeritus)
Ph.D. University of London
B.Sc. University of London
Department of Mathematics
The University of Western Ontario
Middlesex College Rm. 209
London, ON, Canada
N6A 5B7
tel: (519) 661-2111 ext. 89434
email: zamir@uwo.ca
Research Interests
- Blood Flow
- Theoretical Biology
- Biomechanics
Recent Publications
-
-
-
- Brimacombe C, Corless RM, Zamir M, 2023. Elliptic cross sections in blood flow regulation. AIMS Mathematics, 8(10): 23108–23145. DOI:10.3934/math.20231176./
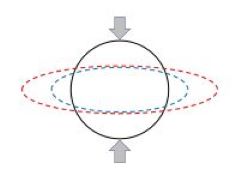
Pulsatile blood flow in a vessel of circular cross section is governed by a Bessel equation with a well known solution in terms of Bessel functions. If on the other hand the vessel has an elliptic cross section, as under pathological circumstances where the vessel is compressed by surrounding tissue, the flow is found to be governed by a Mathieu equation with a less known and less easy to handle solution in terms of Mathieu functions. Aside from the application to blood flow, however, this transition from circular to elliptic geometry represents a puzzle. From the viewpoint of geometry the transition is seemingly smooth/continuous, yet from the viewpoint of the equations governing the flow and the form of the solutions the transition seems surprisingly discontinuous.
- Brimacombe C, Corless RM, Zamir M, 2023. Elliptic cross sections in blood flow regulation. AIMS Mathematics, 8(10): 23108–23145. DOI:10.3934/math.20231176./
-
-
-
-
-
- Moir ME, Klassen SA, Zamir M, Hamner JW, Tan CO, Shoemaker JK, 2023. Regulation of cerebrovascular compliance compared with forearm vascular compliance in humans: a pharmacological study. American Journal of Physiology Heart and Circulatory Physiology 324: H100–H108, 2023. First published December 2, 2022; doi:10.1152/ajpheart.00377.
- Brimacombe C, Corless RM, Zamir M, 2021. Computation and Applications of Mathieu Functions: A Historical Perspective. SIAM REVIEW 63(4):653–720. DOI.10.1137/20M135786X
- Moir ME, Vermeulen TD, Smith SO, Woehrle E, Matushewski BJ, Zamir M, Shoemaker JK, 2021. Vasodilatation by carbon dioxide and sodium nitroglycerin reduces compliance of the cerebral arteries in humans. Experimental Physiology, 106, 1679–1688. doi.org/10.1113/EP089533
- Zamir M, Nelson DM, Ginosar Y, 2021. Hemodynamic Consequences of Incomplete Uterine Spiral Artery Transformation in Human Pregnancy, with Implications for Placental Disfunction and Preeclampsia. Journal of Applied Physiology 130:457-465. Doi:10.1152/japplphysiol.00504.
- Moir ME, Klassen SA, Zamir M, Shoemaker JK, 2020. Rapid changes in vascular compliance contribute to cerebrovascular adjustments during transient reductions in blood pressure in young, healthy adults. Journal of Applied Physiology 129: 27-35. doi:10.1152/japplphysiol.00272.
- Ritman EL, Vercnocke AJ, Zamir M, 2019. Probing the Depth of the Myocardium: Vasculature, Transit Time, and Perfusion Within the Left Ventricular Wall. Annals of Biomedical Engineering. doi.org/10.1007/s10439-019-02208-1
- Zamir M, Nelson DM, Ginosar Y, 2018. Geometric and Hemodynamic Characterization of Uterine Spiral Arteries: The Concept of Resistance Reserve. Placenta. 68:59-64. doi.org/10.1016/j.Placenta.2018.06.006./
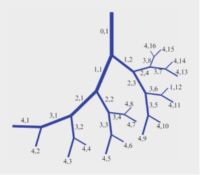
In the human cardiovascular system, blood supply from the heart to every part of the body is facilitated by a massive vascular branching system characterised by repeated bifurcations of a main vessel segment dividing into two branches, then each of the two branches in turn diving into two branches and so on. It has been amply shown that the diameters of the two branch segments and their branching angles are such as to provide optimum passage of blood flow through the bifurcation and the least distance of that flow to its intended destination. Vessel segments between successive bifurcations are short and straight.
Yet, during pregnancy, in the utero-placental blood supply system from mother to fetus, we find SPIRAL ARTERIES. The coiled geometry of spiral arteries in this system is a hemodynamic enigma. it contradicts the vascular branching rules found everywhere else in the cardiovascular system.
-
- Zamir M, Moir ME, Klassen SA, Balestrini CS, Shoemaker JK, 2018. Cerebrovascular Compliance Within the Rigid Confines of the Skull. Frontiers in Physiology. doi.org/10.3389/fphys.2018.00940.
- Onaizah O, Poepping TL, Zamir M, 2017. A model of blood supply to the brain via the carotid arteries: Effects of obstructive vs. sclerotic changes. Medical Engineering and Physics 49:121-30.
- Betti MI, Wahl LM, Zamir M, 2017. Reproduction Number and Asymptotic Stability for the Dynamics of a Honey Bee Colony with Continuous Age Structure. Bulletin of Mathematical Biology 79:1586–1611. DOI 10.1007/s11538-017-0300-7.
- Zamir M, Badrov MB, Olver TD, Shoemaker JK, 2017. Cardiac Baroreflex Variability and Resetting During Sustained Mild Effort. Frontiers in Physiology 8:246. doi:10.3389/fphys.2017.00246.
- Betti M, LeClair J, Wahl LM, Zamir M, 2017. Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies. Insects 2017, 8, 31; doi:10.3390/insects8010031.
- Olver TD, Reid SM, Smith AR, Zamir M, Lemon PWR, Laughlin MH, Shoemaker JK, 2016. Effects of acute and chronic interval sprint exercise performed on a manually propelled treadmill on upper limb vascular mechanics in healthy young men. Physiological Reports 4 (13), 2016, e12861, doi: 10.14814/phy2.12861
- Betti MI, Wahl LM, Zamir M, 2016. Age structure is critical to the population dynamics and survival of honeybee colonies. Royal Society Open Science 3: 160444. http://dx.doi.org/10.1098/rsos.160444
-

Hemo-Dynamics. Springer, New York, 2016.
It is remarkable how readily we accept the proposition that blood is the essence of life, our life. Yet we miss the point. The essence of life is not blood, it is blood flow. When the heart stops beating, the body dies not because of lack of blood but because of lack of blood flow.
Because of the pulsatile nature of blood flow, the integrity of blood supply to any destination within the body depends not only on the integrity of the blood vessels involved but also on the state of oscillatory dynamics of the flow. A disruption in blood supply can result from a derangement in the dynamics of the flow (dynamic pathology) in the same way that it can result from an obstruction in a blood vessel (structural pathology).
Unlike structural pathologies, dynamic pathologies do not leave a “footprint” after they have been resolved or after they have incurred their damage. Following an ischemic event, for example, whether in the heart or in the brain, indeed following a failure or discrepancy in blood supply to any other organ, only structural pathologies can usually be found. Any dynamic pathologies that may have been involved are not in evidence because they are no longer at play.
-
-
-
Zamir M, Verenocke AJ, Edwards PK, Anderson JL, Jorgensen SM, Ritman EL, 2015. Myocardial Perfusion: Characteristics of Distal Intramyocardial Arteriolar Trees. Annals of Biomedical Engineering 43:2771-2779. DOI 10.1007/s10439-015-1325-4
-
Kember G, Ardell JL, Armour JA, Zamir M, 2014. Vagal Nerve Stimulation Therapy: What Is Being Stimulated? PLoS ONE 9(12): e114498. doi:10.1371/journal.pone.
-
Betti MI, Wahl LM, Zamir M, 2014. Effects of Infection on Honey Bee Population Dynamics: A Model. PLoS ONE 9(10): e110237. doi:10.1371/journal.pone.
-
Nielson1 CA, Frances1 MF, Fitzgeorge L, Prapavessis H, Zamir M, Shoemaker JK, 2014. Impact of a smoking cessation lifestyle intervention on vascular mechanics in young women. Applied Physiology, Nutrition, and Metabolism, Published on the web 16 January 2014, 10.1139/apnm-2013-0272
-
Zamir M, Coverdale NS, Barron CC, Sawicki CP, Shoemaker JK, 2014. Baroreflex variability and “resetting”: A new perspective. Journal of Biomechanics doi.org/10.1016/j.jbiomech.2013.09.03.
-
Kember G, Armour JA, Zamir M, 2013. Neural Control Hierarchy Of The Heart Has Not Evolved To Deal With Myocardial Ischemia. Physiological Genomics 45:638-644.
-
Beighley PE, Zamir M, Wentz1 RJ, Koch LG, Britton SL, Ritman EL, 2013. Vascularity of Myocardium and Gastrocnemius Muscle in Rats Selectively Bred for Endurance Running Capacity. Physiol Genomics 45:119–125.
-
Kember G, Armour JA, Zamir M, 2013. Dynamic neural networking as a basis for plasticity in the control of heart rate. Journal of Theoretical Biology 317:39–46.
-
Zamir M, Twynstra J, Vercnocke AJ, Welch I, Jorgensen SM, Ritman EL, Holdsworth DW, Shoemaker JK, 2012. Intrinsic microvasculature of the sciatic nerve in the rat. Journal of the Peripheral Nervous System 17:377–384.
-
Zamir M, Kimmerly DS, Shoemaker JK, 2012. Cardiac mechanoreceptor function implicated during premature ventricular contraction. Autonomic Neuroscience: Basic and Clinical 167:50–55.
-
Frances MF, Goswami R, Rachinsky M, Craen R, Kiviniemi AM, Fleischhauer A, Steinback CD, Zamir M, Shoemaker JK, 2011. Adrenergic and myogenic regulation of viscoelasticity in the vascular bed of the human forearm. Experimental Physiology 96:1129–1137.
-
Hodis S, Zamir M, 2011. Coupled radial and longitudinal displacements and stresses within the arterial wall in pulsatile flow under tethered and free-wall conditions. Physical Review E: 83(5). DOI:10.1103/PhysRevE.83.051923.
-
Kline TL, Zamir M, Ritman EL 2011. Relating Function to Branching Geometry: A Micro-CT Study of the Hepatic Artery, Portal Vein, and Biliary Tree. Cells Tissues Organs. DOI:10.1159/000323482
-
Hodis S, Zamir M, 2011. Mechanical events within the arterial wall under the forces of pulsatile flow: a review. Journal of the Mechanical Behavior of Biomedical Materials. DOI:10.1016/j.jmbbm.2011.01.005
-
Kember GC, Armour JA, Zamir M, 2011. Neural Control of Heart Rate: The Role of Neuronal Networking. Journal of Theoretical Biology 277:41–47. DOI:10.1016/j.jtbi.2011.02.01
-
Hodis S, Zamir M, 2011. Pulse Wave Velocity as a Diagnostic Index: The Pitfalls of Tethering versus Stiffening of the Arterial Wall. Journal of Biomechanics 44:1367-73. DOI:10.1016/j.jbiomech.2010.12.029
-
Zamir M, Goswami R, Liu L, Salmanpour A, Shoemaker JK, 2011. Myogenic activity in autoregulation during low frequency oscillations. Autonomic Neuroscience: Basic and Clinical 159:104-110. DOI:10.1016/j.autoneu.210.07.029
-
Kline TL, Zamir M, Ritman EL, 2010. Accuracy of Microvascular Measurements Obtained from Micro-CT Images. Annals of Biomedical Engineering 38:2851-2864. DOI:10.1007/s10439-010-0058-7
-
Zamir M, Moore JE Jr, Fujioka H, Gaver DP III, 2010. Biofluid Mechanics of Special Organs and the Issue of System Control. Annals of Biomedical Engineering 38:1204-1215. DOI:10.1007/s10439-010-9902-z
-
Hodis M, Zamir M, 2009. Arterial wall tethering as a distant boundary condition. Physical Review E 80:051913.
-
Zamir M, Norton K, Fleischhauer A, Frances MF, Goswami R, Usselman CW, Nolan RP, Shoemaker JK, 2009. Dynamic responsiveness of the vascular bed as a regulatory mechanism in vasomotor control. The Journal of General Physiology. DOI: 10.1085/ jgp.200910218.
-
Hodis S, Zamir M, 2009. Mechanical events within the arterial wall: The dynamic context for elastine fatigue. Journal of Biomechanics 42:1010–1016.
-
Hodis S, Zamir M, 2008. Solutions of the Maxwell viscoelastic equations for displacement and stress distributions within the arterial wall. Physical Review E 78:021914.
-
Dong Y, Beighley PE, Eaker DR, Zamir M, Ritman EL, 2008. Intramyocardial capillary blood volume estimated by whole-body CT – validation by micro-CT. Proc SPIE, Medical Imaging 6916:691624-1 — 691624-6, 2008
-
-


The Physics of Coronary Blood Flow. Springer, New York, 2005.
While the heart, like other parts of the body, is subject to genetic disorders and infectious diseases, only a very small proportion of heart failures are caused by such conditions. In all other cases a lack of blood supply to the heart muscle, or a lack of fuel which the muscle needs for doing its pumping work, is the principal cause of heart failure.
Yet, “heart disease” is the term most widely used in association with heart failure. The term is somewhat misleading because it suggests that the failure is due to a disease of the heart itself. The situation is not unlike that of using the term “malfunction” to describe a car engine failure caused by lack of fuel.
One of the characteristic features of the coronary circulation (the vascular system responsible for providing the heart with its own blood supply) is its capacity to increase blood flow to the heart “on demand” by as much as five or six times normal flow rate, a feature generally referred to as “coronary flow reserve”. The conundrum of coronary flow reserve is that it may “mask” the gradual shortfall in coronary blood flow at the initial stages of coronary heart disease and thereby prevent the remedial mechanism of vascular restructuring from being triggered. At the latter stages of the disease the capacity of coronary flow reserve is largely depleted and the mechanism of vascular restructuring may not have the time required for its slow course of action. Physical conditioning (exercise) may forestall this course of events by deliberately and regularly triggering the mechanism of vascular restructuring while coronary flow reserve is still intact.

The Physics of Pulsatile Flow. Springer-Verlag, New York, 2000.
Flow in a tube is the most common fluid dynamic phenomenon in biology. Its evolution as a tool in biology is so closely intertwined with the evolution of living organisms that it is difficult to view the two as separate from each other. Not even in the realm of science fiction can we imagine the evolution of a living organism of any degree of complexity without the facility of flow in tubes.
When flow is steady, the phenomenon is particularly simple because only two forces are at play: the pressure difference between the two ends of the tube, which acts as a driving force, and viscous shear between the fluid and the tube wall, which acts as a retarding force. When flow is pulsatile, however, the pressure difference between the two ends of the tube varies periodically in time... This introduces other forces and other variables and the phenomenon becomes considerably more complicated particularly when the tube wall is not rigid.
Because of this added complexity, in both mathematics and physics, for far too long the subject of pulsatile blood flow has been practically inaccessible to those who need it most. My deepest hope is that in this book I have made the subject somewhat more accessible, and this I owe largely to the clinical colleagues I have worked with over the years, and to the wonderful students of all stripes who have asked questions.

